
PMR1 is the S. cerevisiae homolog of the SPCA (Secretory Pathway Calcium ATPase) family. This family of proteins is thought to be structurally similar to the SERCA family, containing ten transmembrane domains, with the caiton binding site located within the cation transport pathway made up of M4, M5, M6 and M8. The large degree of similarity has allowed us to use homology modeling to create an in silicon model of the protein which will serve as the basis for further biochemical characterization. Unlike the SERCA, which transports two calcium cations per ATP hydrolyized, the SPCA family may only transport one calcium or manganese ion (similar to the PMCA family) because of lack of conservation in cation coordination amino acids in M5. PMR1 (and the human homolog) are not thapsigargin sensitive.
The SPCA protein is structurally defined by ten transmembrane domains, a large beta-sheet "actuator" domain between M2 and M3, and the large ATPase domain between M4 and M5. The ATPase domain is broken into sub-domains consisting of the nucleotide binding (N) and phosphorylation domain (P).The actual phosphorylation site is an aspartic acid, in this particular enzyme it is D371.
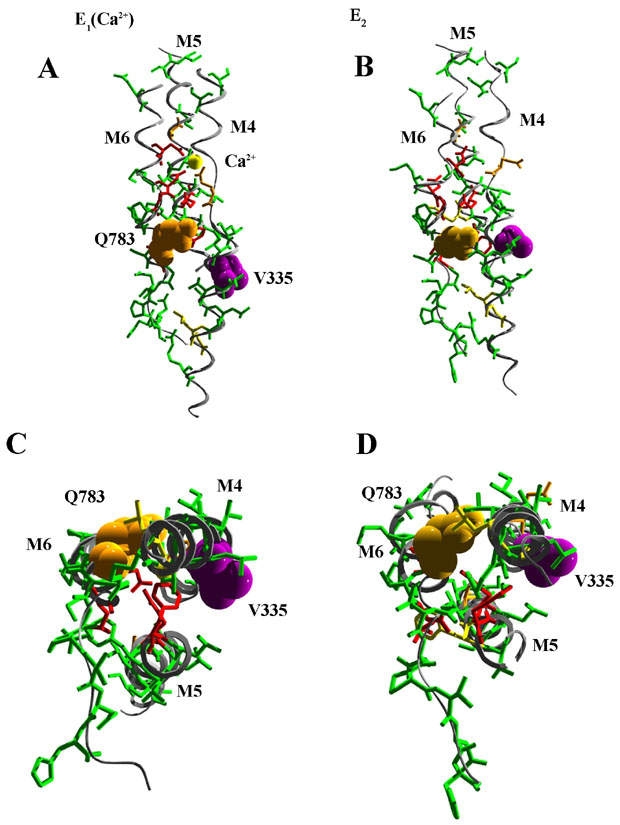
Several site directed mutagenesis studies have been initiated to investigate the functionality of individual amino acids within the transmembrane domains and their contibution to either calcium and/or manganese ion transport. Site directed mutagenesis also has identified individual side chains that determine ion selectivity between calcium and manganese. Mutant Q783A, V335G, and V335Q are termed selectivity mutations since these mutations allow for calcium transport but not manganese. Interestingly, mutations at V335 (purple) are able to compensate for the selectivity mutations in Q783 (orange) suggesting some sort of intra-membrane interactions. This is illustrated in the figure above where helices M4, M5 and M6 (and Ca2+; yellow sphere) are shown perpendicular to the plane of the membrane (A and B) or in cross-section from the cytoplasmic side of the membrane (C and D) in the E1 (Ca2+-bound; A and C) and E2 (B and D) conformations (ref). Residues contributing to the Ca2+ binding site in the E1 structure are displaced in the E2 conformation. Note the movements in the cytoplasmic halves (C and D) of M4 and M6 that bring V335 and Q783 in proximity in the E2 conformation.