JHU Department Affiliation: Cell Biology
Education: B.S. , Goucher College, Baltimore, M.D.
Telephone Number: 410-955-2337
Fax Number: 410-955-1013
Email Address: lnyasae@bs.jhmi.edu

| Lydia
Nyasae
JHU Department Affiliation: Cell Biology Education: B.S. , Goucher College, Baltimore, M.D. Telephone Number: 410-955-2337 Fax Number: 410-955-1013 Email Address: lnyasae@bs.jhmi.edu
|
|
Transcytosis is dependent on cholesterol and glycosphingolipids in polarized hepatocytes.
Lydia K. Nyasae, Ann L. Hubbard,
and Pamela L. Tuma
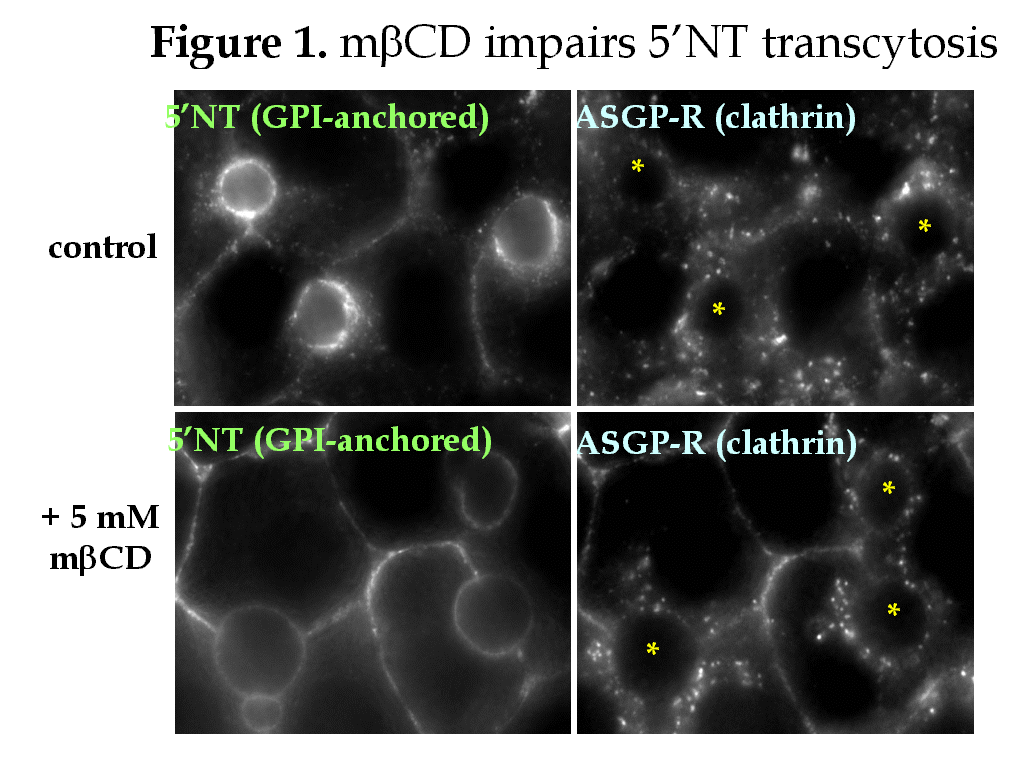
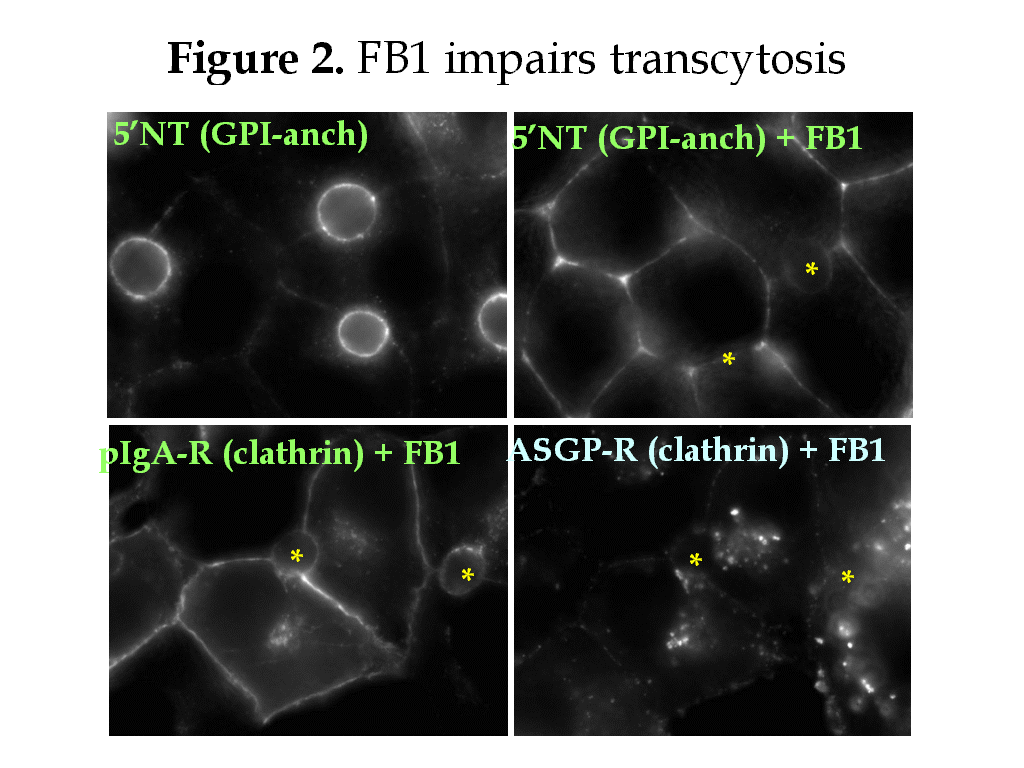
The intrinsic properties of glycosphingolipids and cholesterol promote their assembly into specialized domains called rafts. The rafts are thought to form in the TGN where they recruit apically-destined proteins and mediate their direct transport to the apical plasma membrane (PM). In hepatocytes, single transmembrane domain (TMD) and glycolipid (GPI)-anchored apical proteins take the “indirect” route to the apical PM. They are transported from the TGN to the basolateral PM where they are endocytosed and transcytosed to the apical surface. Do rafts sort hepatic apical proteins from the TGN? In the transcytotic pathway? We examined the Triton X-100-insolubility (a biochemical property of rafts) of domain-specific proteins in WIF-B cells. All basolateral proteins examined, including recycling receptors, were detergent soluble. Of the apical residents examined, only CD59 and 5’nucleotidase (GPI-anchored proteins) and aminopeptidase N (a single TMD protein) were detergent-insoluble. This result and the examination of the acquisition of detergent-insolubility of newly synthesized proteins indicated that rafts are not solely responsible for sorting apical residents from the TGN. To determine whether rafts sort proteins in the transcytotic pathway, we monitored the basolateral to apical transport of apical proteins in WIF-B cells treated with cyclodextrin (to deplete cholesterol) or fumonisin B1 (to deplete glycosphingolipids).
 |
 |
|
|
Both drugs inhibited transcytosis of all apical residents examined whereas they only partially inhibited fluid phase uptake of HRP or clathrin-mediated internalization of transferrin and asialoglycoprotein receptors. Although pIgA-R is also internalized via a clathrin-mediated pathway, surprisingly its transcytosis was nearly completely inhibited in treated cells. Interestingly, the solubility of the apical residents did not correlate with cholesterol depletion (measured by thin layer chromatography) or with the inhibition of transcytosis. Thus, other factors besides raft components are contributing to the insolubility of the transcytosing proteins.