JHU Department Affiliation: Cell Biology
Education: PhD 1999 Rutgers University/UMDNJ
Telephone Number: 410-955-2337
Fax Number: 410-955-4129
Email Address: lkallay@jhmi.edu

| Laura
M Kallay
JHU Department Affiliation: Cell Biology Education: PhD 1999 Rutgers University/UMDNJ Telephone Number: 410-955-2337 Fax Number: 410-955-4129 Email Address: lkallay@jhmi.edu
|
|
Localization
of scribble in polarized
hepatocytes
Laura
M. Kallay, Lelita T. Braiterman, Pamela L. Tuma, Ya-Hui Chen and Ann L. Hubbard
Scribble is a large, multi-domain protein important for the establishment
and maintenance of epithelial cell polarity in D. melanogaster and C. elegans;
however, its role in mammalian epithelial cells is largely unknown.
Scribble is a LAP (LRR (leucine rich repeats) and PDZ (PSD-95/Disc
Large/ZO-1)) family member. Family
members share a structural organization consisting of sixteen amino-terminal
LRRs followed by a LAP-specific domain of unknown function and either one or
four carboxyl-terminal PDZ domains. We have examined the subcellular location of
scribble in polarized hepatocytes using morphological, biochemical, and
molecular approaches. In WIF-B
cells, scribble was found in multiple locations by indirect immunofluorescence:
the basolateral plasma membrane, the tight junction region, in punctae at the
cell-substrate, and within the cytoplasm. Biochemically,
scribble was greater than 90% membrane-associated. Scribble was also present in purified rat liver plasma
membrane sheets. When the apical
and basolateral domains were separated by sucrose density centrifugation,
scribble distributed to a pelleted fraction, indicating its presence in a high
density complex. Scribble was
insoluble in several different detergents further suggesting its presence in a
membrane-associated multimeric complex.
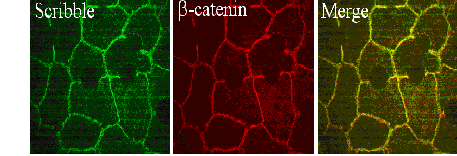
Figure 1
To map the domains that mediate its membrane localization, GFP-tagged scribble mutants were generated and expressed in WIF-B cells using recombinant adenoviruses. The localizations of the GFP-tagged scribble EST, and four progressive truncation mutants, DLRR, DLRR+LAP and the PDZ1-4 + C-Term (the C-terminal half of scribble that containing the four PDZ domains), and PDZ1-4 were similar to that of endogenous scribble: all five mutants were found at basolateral plasma membrane and in the tight junction region. However, the construct encoding the C-terminal region beyond the PDZ domain was soluble. These show that in polarized hepatocytes the C-terminal half of scribble with four PDZ domains plays a role in its PM localization. Current experiments focus on: a) identifying the localization signal(s) in the C-terminal half of scribble; and b) characterizing the proposed plasma membrane complex and identifying interacting molecules.
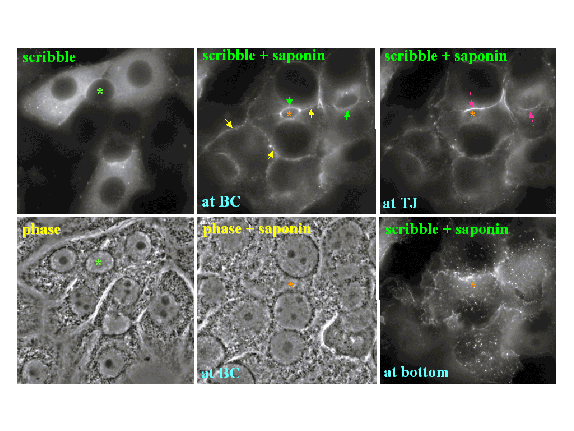
|