| EMBRYONIC POLARITY AND THE SOMA-GERMLINE
DICHOTOMY
Our lab studies the earliest stages of embryogenesis to
understand how single-celled eggs develop into complex multicellular
embryos. We focus on the choice between soma and germline,
one of the first developmental decisions faced by embryos.
Our goal is to identify and characterize the molecular mechanisms
that activate embryonic development, polarize embryos, and
distinguish between somatic and germline cells, using Caenorhabditis
elegans as a model system. Our research program is divided
into three areas:
Oocyte-to-embryo transition
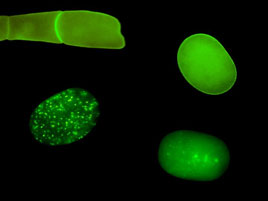
FIGURE 1: Localization of MBK-2 changes during
the oocyte-to-embryo transition. |
The beginning of development is marked by a remarkable transition:
the quiescent oocyte is transformed into a dynamic embryo
ready to differentiate into many cell types. We recently
found that the oocyte-to-embryo transition requires the coordinate
degradation of several oocyte proteins. We identified minibrain
kinase 2 (MBK-2), a member of the evolutionarily conserved
DYRK family of kinases, as a candidate master regulator of
oocyte protein degradation. MBK-2 phosphorylates oocytes
proteins shortly after fertilization during the meiotic divisions.
Surprisingly, progression through the meiotic divisions,
rather than fertilization per se, is what is required to
activate MBK-2 and initiate protein degradation. Premature
entry into meiotic M phase in unfertilized oocytes
is sufficient to relocalize MBK-2 from the cortex to the
cytoplasm and trigger the degradation of oocyte proteins. Our
findings suggest that, in addition to its well-known role
in regulating chromosome dynamics, the meiotic cell cycle
triggers egg-wide developmental changes essential for the
initiation of embryonic development. We are currently investigating
the mechanisms that link the meiotic cell cycle to MBK-2
and the transition from oocyte to embryo.
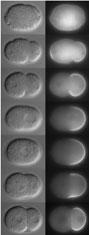
FIGURE 2: PAR-2 dynamics during polarization of the
zygote. |
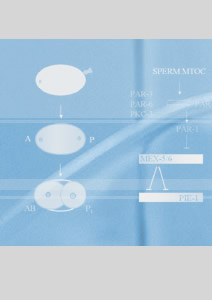
Embryonic polarity
This part of our research seeks to understand how embryos
become polarized along the anterior/posterior axis and how
this spatial information is used to delineate distinct somatic
(anterior) and germline (posterior) domains. A/P polarity
is initiated by a microtubule-organizing center (MTOC) brought
in by the sperm at fertilization. In collaboration with the
Kemphues Lab (Cornell U.), we have found that the primary
effect of the MTOC is to displace polarity regulators
PAR-3, PAR-6 and PKC-3 away from the sperm, allowing PAR-1
and PAR-2 to accumulate on the cortex nearest the sperm.
Using live imaging of GFP-tagged proteins and biochemistry,
we have begun to identify the molecular mechanisms underlying
PAR dynamics. A critical step is phosphorylation by PKC-3
of PAR-1 and PAR-2 on residues essential for cortical localization.
Our findings support a model where reciprocal inhibitory
interactions between PAR proteins polarize the zygote by
reinforcing an initial asymmetry in PKC-3.
Soma-germline dichotomy
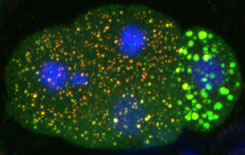
FIGURE 3: Somatic (3 cells on left) and germline (1
cell on right) blastomeres contain different types
of RNP granules. |
The
zygote undergo a series of asymmetric divisions to generate
somatic and germline blastomeres. Somatic blastomeres degrade
maternal RNAs and activate transcription soon after their
separation from the germ lineage. In contrast, germline blastomeres
maintain maternal RNAs and delay the activation of mRNA transcription
until after gastrulation. The difference in transcriptional
activity is due to PIE-1, a global transcriptional repressor
that segregates with the germline blastomeres. PIE-1 interferes
with phosphorylation of the carboxy terminal domain (CTD)
repeats of RNA polymerase II. In collaboration with
the Blackwell lab (Harvard U.), we have shown that a PIE-1
inhibits transcription in part via a motif that resembles
the CTD repeats.
The mechanisms that lead to a difference
in maternal RNA stability between somatic and germline blastomeres
are less understood. We have found that this difference correlates
with distinct classes of cytoplasmic ribonucleoprotein particles
(RNPs) found in the two cell types. Somatic blastomeres
contain granules similar to the RNA degradation centers of
yeast and mammalian cells (P-bodies). Germline blastomeres
contain related, but compositionally distinct, larger granules
(Fig. 3). We are currently investigating the function of
these germline-specific structures. RNA
binding proteins are common among regulators of germ cell development in many
organisms, raising the possibility that germ cells preferentially use post-transcriptional
mechanisms to regulate gene expression. To address this question, we are
developing methods to systematically characterize the expression of germline
genes.
We gratefully acknowledge support from the National Institutes
of Health and the Howard Hughes Medical Institute.
|